Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Brief introduction of Hexanophenone CAS 942-92-7 Chemical name:Phenylhexanone CAS No.:942-92-7 Synonyms: Amyl Phenyl Ketone Caprophenone Molecular Formula: C12H16O Molecuar Weight:176.25 EINECS: 213-394-6 Sell Hexanophenone Cas 942-92-7
- CAS No.
- 942-92-7
- Chemical Name:
- Hexanophenone
- Synonyms
- Hexaphenone;1-Phenyl-1-hexanone;N-AMYL PHENYL KETONE;1-Hexanone, 1-phenyl-;CAPROPHENONE;HEXANOPHENONE;N-HEXANOPHENONE;Hexanophenone>Hexanophenone 99%;Hexanophenone,98%
- CBNumber:
- CB7221671
- Molecular Formula:
- C12H16O
- Molecular Weight:
- 176.25
- MDL Number:
- MFCD00009512
- MOL File:
- 942-92-7.mol
- MSDS File:
- SDS
| Melting point | 25-26 °C (lit.) |
|---|---|
| Boiling point | 265 °C (lit.) |
| Density | 0.958 g/mL at 25 °C (lit.) |
| refractive index | n20/D 1.5105(lit.) |
| Flash point | >230 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| form | Liquid After Melting |
| color | Clear light yellow |
| BRN | 1908667 |
| InChI | InChI=1S/C12H16O/c1-2-3-5-10-12(13)11-8-6-4-7-9-11/h4,6-9H,2-3,5,10H2,1H3 |
| InChIKey | MAHPVQDVMLWUAG-UHFFFAOYSA-N |
| SMILES | C(C1=CC=CC=C1)(=O)CCCCC |
| LogP | 3.790 (est) |
| CAS DataBase Reference | 942-92-7(CAS DataBase Reference) |
| EPA Substance Registry System | Hexanophenone (942-92-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29143900 | |||||||||
| NFPA 704 |
|
Hexanophenone price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | H12455 | Hexanophenone 99% | 942-92-7 | 5g | $45 | 2023-06-20 | Buy |
| Sigma-Aldrich | H12455 | Hexanophenone 99% | 942-92-7 | 25g | $85.9 | 2023-06-20 | Buy |
| TCI Chemical | H0116 | Hexanophenone >98.0%(GC) | 942-92-7 | 25g | $71 | 2023-06-20 | Buy |
| TCI Chemical | H0116 | Hexanophenone >98.0%(GC) | 942-92-7 | 500g | $689 | 2023-06-20 | Buy |
| Alfa Aesar | A14712 | Hexanophenone, 98% | 942-92-7 | 5g | $33 | 2023-06-20 | Buy |
Hexanophenone Chemical Properties,Uses,Production
Chemical Properties
clear light yellow liquid after melting
Uses
Caprophenone is an inhibitor of carbonyl reductase activity in pig’s heart cytosol. A useful research chemical.
Synthesis Reference(s)
The Journal of Organic Chemistry, 44, p. 3585, 1979 DOI: 10.1021/jo01334a033
Synthetic Communications, 8, p. 59, 1978 DOI: 10.1080/00397917808062184
Tetrahedron Letters, 24, p. 3677, 1983 DOI: 10.1016/S0040-4039(00)88199-X
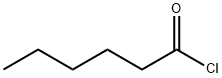
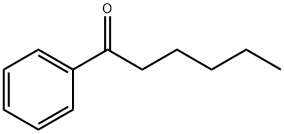
Hexanophenone Preparation Products And Raw materials
Raw materials
Preparation Products
Hexanophenone is a chemical compound used as a precursor in the synthesis of pharmaceuticals and fine chemicals. It can also be used as a reagent in organic chemistry, and as an intermediate in the production of fragrances and flavors. This product comes in 1x5g packaging, should be handled with care and stored in a dry place at room temperature. Hexanophenone is flammable and harmful if ingested or inhaled, so proper safety precautions must be taken when handling it. Environmental impact information for this product is not available.
| Description | Hexanophenone (C6H5COC6H5) is an aromatic ketone that is widely used as a synthetic intermediate in the production of a variety of chemicals and materials. It is also used as a starting material in the synthesis of pharmaceuticals and other compounds. Hexanophenone has a wide range of applications, including in the manufacture of dyes, fragrances, and other organic compounds. |
| Synthesis Method | Hexanophenone is produced through the condensation of benzaldehyde and acetone, which is catalyzed by a base such as sodium hydroxide. The reaction involves the formation of an enolate intermediate, which then undergoes a nucleophilic attack by the electrophilic benzaldehyde. The reaction is typically carried out in an aqueous medium, and the resulting product is purified by recrystallization or distillation. |
| Synthesis Method Details | Design of the Synthesis Pathway The synthesis of Hexanophenone can be achieved through the Friedel-Crafts acylation reaction of benzene with hexanoyl chloride.Starting Materials Benzene, Hexanoyl chloride, AlCl3, Anhydrous etherReaction Add AlCl3 to anhydrous ether and stir to form a Lewis acid catalyst. Add hexanoyl chloride dropwise to the Lewis acid catalyst while stirring. Add benzene dropwise to the reaction mixture while stirring. Heat the reaction mixture to reflux for several hours. Allow the reaction mixture to cool and then pour it into ice-cold water. Extract the organic layer with a solvent such as diethyl ether. Dry the organic layer with anhydrous sodium sulfate. Filter the organic layer and evaporate the solvent to obtain the crude product. Purify the crude product through recrystallization or column chromatography. |
| Scientific Research Application | Hexanophenone has been extensively studied in the fields of organic synthesis, medicinal chemistry, and biochemistry. It has been used as a key intermediate in the synthesis of a variety of drugs, including the antifungal agent ketoconazole and the anti-inflammatory agent indomethacin. In addition, it has been used as a starting material in the synthesis of a variety of other compounds, including the antimalarial drug chloroquine and the anti-HIV drug zidovudine. |
| Mechanism of Action | Hexanophenone is a highly reactive compound, and its reactivity is due to its ability to form an enolate intermediate. This intermediate is then attacked by a nucleophile, such as a benzaldehyde molecule, which leads to the formation of a new bond. This reaction is known as a nucleophilic addition reaction. |
| Biochemical and Physiological Effects | Hexanophenone has been shown to have a variety of biochemical and physiological effects. In vitro studies have demonstrated that it can inhibit the activity of enzymes involved in the metabolism of drugs, such as cytochrome P450 enzymes. In addition, it has been shown to possess anti-inflammatory, anti-bacterial, and anti-fungal properties. |
| Advantages and Limitations for Lab Experiments | Hexanophenone is a highly reactive compound, and as such, it has a number of advantages and limitations when used in laboratory experiments. One of the main advantages of using hexanophenone is that it can be used to synthesize a wide range of compounds in a relatively short amount of time. However, the reactivity of the compound can also be a limitation, as it can lead to the formation of undesired side products. |
| Future Directions | The potential applications of hexanophenone are vast, and there are a number of areas that could be explored in the future. One of the most promising areas is the development of new drugs and other compounds that can be synthesized using hexanophenone as a starting material. In addition, further research could be conducted on the biochemical and physiological effects of hexanophenone, as well as its potential toxicity. Finally, the synthesis of hexanophenone can be further optimized to reduce the amount of time and resources required for its production. |
| CAS RN | 942-92-7 |
| Product Name | Hexanophenone |
| Molecular Formula | C12H16O |
| Molecular Weight | 176.25 g/mol |
| IUPAC Name | 1-phenylhexan-1-one |
| InChI | InChI=1S/C12H16O/c1-2-3-5-10-12(13)11-8-6-4-7-9-11/h4,6-9H,2-3,5,10H2,1H3 |
| InChI Key | MAHPVQDVMLWUAG-UHFFFAOYSA-N |
| SMILES | CCCCCC(=O)C1=CC=CC=C1 |
| Canonical SMILES | CCCCCC(=O)C1=CC=CC=C1 |
| Boiling Point | 265.0 °C |
| Melting Point | 27.0 °C |
| Other CAS RN | 942-92-7 |
| Pictograms | Irritant |
| Origin of Product | CHINA |
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
Sell Hexanophenone Cas 942-92-7
-
Acros Organics AC120720250 Hexanophenone 98% (25g) – Cole …
Buy Acros Organics AC120720250 Hexanophenone 98% (25g) and more from our comprehensive selection of Hexanophenone
-
Buy Hexanophenone, 1x500g | ChemDirect
Buy Hexanophenone, 1x500g online at low prices, delivered in just a few days!
-
Buy Hexanophenone, 1x5g | ChemDirect
chemical products. Buy Hexanophenone, 1x5g online at low prices, delivered in just a few days!
-
Hexanophenone for Sale from Quality Suppliers – ECHEMI
WebBuying Hexanophenone on www.echemi.com where you will find large variety of good choices. Suppliers of 47 Hexanophenone registered will be verified by the accurate …
-
Hexanophenone price,buy Hexanophenone – chemicalbook
WebHexanophenone price,buy Hexanophenone,Hexanophenone Manufacturers ,Hexanophenone Suppliers Directory – Find a Hexanophenone Manufacturer and …
-
Buy Hexanophenone – 942-92-7 | BenchChem
Benchchem offers qualified products for CAS No. 942-92-7(Hexanophenone), please inquire us for more detail.
-
cas 942-92-7|| where to buy Hexanophenone – CHEMENU
cas 942-92-7|| where to buy Hexanophenone, Chemenu is research-based manufacturer of pharmaceutical intermediates and fine chemicals offering Animo acid and peptide, …
-
Buy Hexanophenone
t Hexanophenone. Chat now for more business.





Reviews
There are no reviews yet.